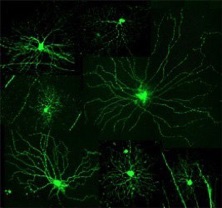
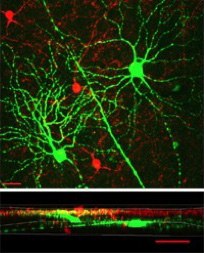
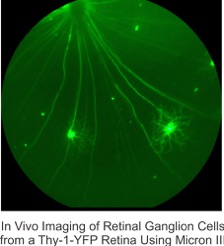
My laboratory has two main research interests. The first is to understand how retinal ganglion cells (RGCs) acquire their dendritic morphology during postnatal development. The complex yet characteristic dendritic structures of RGCs determine their functional properties. For example, morphological specializations in the laminar patterns of RGC dendrites reflect the functional separation of ON and OFF pathways. We have discovered that bi-laminated ON-OFF RGCs and mono-laminated ON or OFF RGCs mature at different rates. After eye-opening, a subpopulation of ON-OFF RGCs are converted into ON or OFF RGCs. The ON RGCs, including those converted from the ON-OFF RGCs, continue to elaborate their dendritic structures. Both of these processes require visual experience. We are specifically interested in the molecular mechanisms underlying the postnatal development of RGC dendritic structure. We focus on the roles of two neurotrophins, Brain-derived neurotrophic factor (BDNF) and Neurotrophin 3 (NT-3), known to regulate the survival, development, and function of neurons in the brain. We have generated or acquired several lines of transgenic mice in which the dendritic structures of RGCs are delineated in high resolution and neurotrophin signaling can be manipulated temporally or spatially to study how BDNF and NT-3 signaling affects RGC dendritic development.
The second is to determine how neurotrophins can be used to protect RGCs in glaucoma. Glaucoma is a group of neurodegenerative diseases characterized by progressive changes of optic nerve head cupping, RGC dendritic and axonal degeneration, visual field deficits, and RGC death. Unfortunately, how an RGC degenerates before it dies is poorly understood. There is growing evidence that BDNF acts as a neuroprotective agent to maintain RGC health, but little is known about whether and how BDNF protects retinal circuitry and visual function against the insults of glaucoma. Combining mouse genetics, imaging, physiological techniques and behavioral assays, we investigate the structural and functional degeneration of RGCs, and the underlying neuroprotective mechanisms of BDNF in glaucoma. We have established a mouse model of laser-induced ocular hypertension to mimic human high-tension glaucoma. Using an inducible Cre-mediated recombination system, we overexpress BDNF in RGCs at different time points before and after the induction of ocular hypertension to investigate whether and how overexpression of BDNF protects RGC structure and visual function in ocular hypertensive eyes. Taken together, our studies will provide a comprehensive assessment of the nature of glaucomatous damage on RGCs and the underlying neuroprotective mechanisms of BDNF and herald new therapies for glaucoma patients.